To Make Novel Atomic Properties, Yale Engineers Change Electron Trajectories
How do you make nickel look and behave like copper?
A team of scientists at Yale University has done just that by developing a novel technique to artificially alter a material’s atomic properties by substantially modifying the orbital properties of electrons. The electrons can also be tunably configured in orbital patterns with unique magnetic, superconductive, and optical properties.

“With this method, we can engineer the electron orbitals of nickel to instead be nearly identical to copper,” said Charles Ahn, the William K. Lanman Jr. Professor of Mechanical Engineering & Materials Science, Applied Physics, and Physics, and co-principal investigator of the research published January 12 in Physical Review Letters. “The fundamental atomic property of each element is determined, in part, by the electron configuration, so as we alter the electron orbital, these properties also change.”
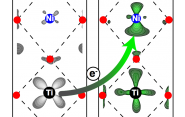
The key is molecular beam epitaxy, a method for growing a crystalline material one atomic layer at a time. The team used the method to create an ordered crystal structure of repeating stacks of atomically thin oxide layers. The stacks are comprised of a titanium-based bottom layer, a transition metal in the second layer (in this case, a nickel-based oxide), and finally an insulating third layer containing aluminum. The result, according to the researchers, is an extremely asymmetric environment around the second layer, which exploits the electrochemistry to cause an electron to move from the titanium to the nickel. The resulting electric field changes the nickel’s orbital shape.

The technique was based on the theoretical calculations of co-principal investigator Sohrab Ismail-Beigi, an associate professor of applied physics, physics, and mechanical engineering & materials science who first generated determined what layering configuration would most strongly and flexibly affect the orbital properties.
“Varying the elements in our fabrication technique can arrange the orbitals in continuous gradations between the configurations found in each element,” said doctoral student Ankit Disa, lead author of the research. “This allows one to transcend the discrete nature of the periodic table, providing fine control over material properties that depend on orbitals.”
As one example, said Disa, the orbital properties could be tuned to the border between a magnetic and a nonmagnetic state; using an electric or magnetic field, the material could then switch easily from magnetic to nonmagnetic and back again. “Our technique makes possible previously unexplored properties,” he said.
Additional authors include Divine Kumah, Andrei Malashevich, Hanghui Chen, and Fred Walker of Yale; Dario Arena of the Brookhaven National Laboratory; and Eliot Specht of the Oak Ridge National Laboratory.

