Researchers distill the facts of a chemical separation process (and upend a decades-old theory)

Since it was developed a few decades ago, the chemical separation process of organic solvent nanofiltration (OSN), has drawn attention for its potential to revolutionize vital industries, including those in fuel, food, and pharmaceuticals.
 Now, a team of researchers from Yale and the University of Wisconsin-Madison has upended the prevailing wisdom on how this rapidly emerging technology works - an insight that could significantly improve the technology. The results of their work are published in Science Advances.
Now, a team of researchers from Yale and the University of Wisconsin-Madison has upended the prevailing wisdom on how this rapidly emerging technology works - an insight that could significantly improve the technology. The results of their work are published in Science Advances.
Organic solvents are liquids that contain carbon and oxygen - examples include alcohols, ethanol, benzene, and ketones. OSN uses membranes with nanosized holes to separate the chemicals. Applications include hydrocarbon separation in oil refining and purifying pharmaceutical ingredients. Because it’s much cheaper and doesn't rely on chemical additives, it’s considered by many to be a potentially better alternative to such traditional technologies as distillation, evaporation, and solvent extraction.
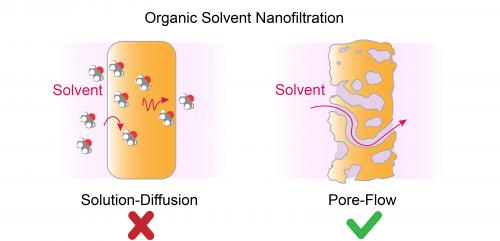
Exactly how it works, though, has been unclear. The most widely held theory posits that OSN relies on solution-diffusion mechanism - that is, a process in which solvent molecules dissolve and diffuse through the membrane from where they’re highly concentrated to wherever there are fewer molecules.
In a previous study, Yale’s Menachem Elimelech and the University of Wisconsin-Madison team used a combination of experiments and computer simulations to negate a decades-long theory on the physics of reverse osmosis, a method for removing salt from seawater. As with OSN, the traditional view attributed reverse osmosis to the solution-diffusion mechanism. Elimelech’s research, though, demonstrated that it was actually changes in pressure within the membranes that pushed the water through the membranes, also known as the pore-flow model.
Since both processes have similarities, Elimelech and the research team applied their skepticism toward the conventional thinking on organic solvent nanofiltration. Using a similar set of experiments and computer simulations, Elimelech and his University of Wisconsin-Madison collaborators showed that the OSN process is driven not by the concentration of solvent molecules but - as in reverse osmosis - by changes in pressure.
One key piece of evidence, said Elimelech, is that the pores of the membranes for organic solvents are large enough to allow the passage of numerous solvent molecules that are held together.
“So it's a cluster,” said Elimelech, the Sterling Professor of Chemical and Environmental Engineering. “And once you have a cluster solvent, it cannot move by diffusion. You need to have a driving force like pressure.”
They also found that for organic solvents, which are more complex than water, friction between the solvent and the polymer membrane plays a significant role. The researchers note that how strongly the solvent binds to the material is intricately linked to how fast the solvent flows through the membrane. In some cases, certain organic solvents can also stretch or compress the polymer material by interacting with it.
With a better understanding of the physics of OSN, scientists can improve the technology of OSN. For instance, Elimelech said, they can use these discoveries to optimize the design of the filtering membranes, thereby enabling a diverse range of separations and clean energy technologies.
Ying Li, an associate professor of mechanical engineering at UW-Madison who led the computer simulations, said that their investigations emphasize the “crucial role played by the disparity between solvent size and membrane pore size in dictating solvent permeance.”
“Fine-tuning the pore size of various dense polymer membranes to approximate the molecular size of unwanted permeants, coupled with the use of a solvent possessing a smaller molecular size, holds the potential for achieving highly selective separations in OSN,” Li said.

