New Clues to How Metallic Glasses Form

For millennia, people have used molten sand and other ingredients to create a vast array of traditional glass products, including beads, vessels, lenses and windows.
 Today, we also have metallic glasses – materials with the strength of metal, but with the pliability of plastic – that are being developed for a broad range of applications: aerospace, robotics, consumer electronics, sporting goods, and biomedical uses. These materials owe their properties to their unique atomic structures: when metallic glasses cool from a liquid to a solid, their atoms settle into a random arrangement and do not crystallize the way traditional metals do.
Today, we also have metallic glasses – materials with the strength of metal, but with the pliability of plastic – that are being developed for a broad range of applications: aerospace, robotics, consumer electronics, sporting goods, and biomedical uses. These materials owe their properties to their unique atomic structures: when metallic glasses cool from a liquid to a solid, their atoms settle into a random arrangement and do not crystallize the way traditional metals do.
Controlling the creation of metallic glasses, however, remains an inexact endeavor informed largely by long experience and intuition. With a new study, published today in Nature Communications, researchers at Yale and the University of Wisconsin-Madison have come closer to taking some of the guesswork out of the formation of these materials.
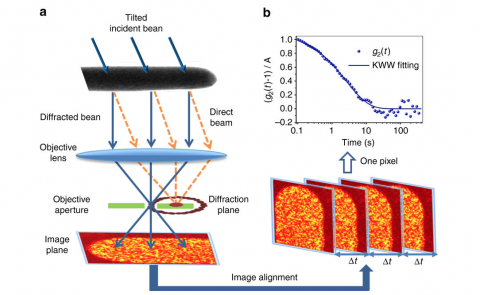
Specifically, the researchers have made significant experimental strides in understanding how, when and where the constantly moving atoms in molten metal "lock" into place as the material transitions from liquid to solid glass. The discovery could help reduce time and costs in developing new metallic glass materials and provide manufacturers greater insight into process design.
The researchers’ work upends a long-held theory that the atoms of metallic glasses move together as the material is forming, accelerating and slowing down in unison as temperatures change. Experiments in recent years have suggested otherwise, but did not provide direct evidence.
“People have speculated that there are different regions of atoms and that the atoms in some regions move quicker than in other regions - these are called spatial heterogeneities,” said one of the study’s authors, Jan Schroers, Yale professor of mechanical engineering & materials science. “But no one had ever seen that directly.”
Drawing on their expertise in electron microscopy and data analysis, though, the researchers have measured how long it takes, on average, for an atom to gain or lose adjacent atoms as its environment fluctuates in the molten liquid.
“Through this technique, we were able to see not only atoms but also how they move around and lock into place,” Schroers said. “We can now quantify it. And it’s quite dramatic – the difference in how these atoms move around is by at least an order of magnitude.”
As metal transitions from molten liquid to solid, its tendency is to form neatly arranged, regularly repeating atomic structures called crystals. In contrast, glass materials have a highly disordered atomic structure. Preventing metal atoms from forming crystals as the material cools relies somewhat on the luck of the draw, which makes processing metallic glass challenging.
"The process that makes a glass and the process that makes a crystal compete with each other, and the one that wins—the one that happens at a faster rate—determines the final product," said Paul Voyles, professor of mechanical engineering at University of Wisconsin-Madison, and lead author of the study.
The researchers’ discovery provides valuable information about the fundamental process through which every glass material—from window glass to plastic bottles to pharmaceutical preparations and many others—transitions from liquid to solid. Ultimately, it could lead to better control in making metallic glasses.
The collaborators are now working to understand how the atomic arrangements differ between the slow and fast parts.
Funding from the National Science Foundation (Nos. DMR-1506564 and DMR-1121288) and from the U.S. Department of Energy Office of Basic Energy Sciences (No. DE SC0004889) supported the research. Other authors on the paper include Pei Zhang, Jason Maldonis of UW-Madison and Ze Liu of Yale University.

