An mRNA COVID vaccine (and potentially more) with nanoparticles, no shot needed
A team of researchers has developed an inhalable vaccine that successfully protects against the COVID virus. It also opens the door to delivering other messenger RNA (mRNA) therapeutics for gene replacement therapy and other treatments in the lungs.
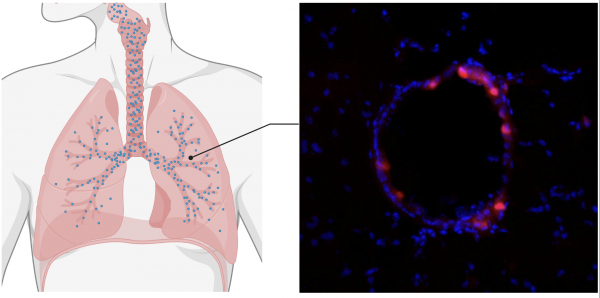 Results of the study, led by Prof. Mark Saltzman, are published in Science Translational Medicine.
Results of the study, led by Prof. Mark Saltzman, are published in Science Translational Medicine.
For the vaccine, the researchers demonstrated that two intranasal doses of the treatment, made with nanoparticles carrying mRNA COVID vaccine, is effective in mice. It also demonstrates that an inhalable delivery system allows for minimally invasive and lung-targeted mRNA delivery, potentially applicable for numerous pulmonary diseases in addition to COVID.
It’s a significant advance since scientists have had trouble creating lung-targeted mRNA therapies. Typically, these therapies have had poor transfection efficiency - that is, only a small fraction of administered nucleic acids make it into cells that lead to expression of the encoded protein. Also, in the past, the nanoparticles that deliver the mRNA have caused inflammation and other problems. The Saltzman group got around this hurdle in part by using a nanoparticle made from poly(amine-co-ester) polyplexes, or PACE, a biocompatible and highly customizable polymer.
Saltzman previously worked with the lab of Akiko Iwasaki, Sterling Professor of Immunology, on what Iwasaki calls a “prime and spike” COVID vaccine delivery system. The “prime” half of the system involves injections of the mRNA vaccine into a muscle - the shot that millions of people have already received. These vaccinations were followed up with familiar spike proteins or spike mRNA that are derived from the coronavirus and are sprayed directly into the nose.
In their new study, the researchers showed that the shot isn’t necessary to provide protection.
“In the new report, there is no intramuscular injection,” said Saltzman, the Goizueta Foundation Professor of Biomedical Engineering, Chemical & Environmental Engineering & Physiology, and a member of Yale Cancer Center. “We just gave two doses, a prime and a boost, intranasally, and we got a highly protective immune response. But we also showed that, generally, you can deliver different kinds of mRNA. So it's not just good for a vaccine, but potentially also good for gene replacement therapy in diseases like cystic fibrosis and gene editing. We used a vaccine example to show that it works, but it opens the door to doing all these other kinds of interventions.”
Without the protective casing of the nanoparticles, the mRNA would quickly deteriorate inside the body. However, developing a nanoparticle for lung-targeted therapies has been tricky. Other attempts to develop an inhalable delivery system for mRNA have met obstacles due to the type of material used for the nanoparticles.
“It's been a challenge trying to take the lipid nanoparticle vaccine delivery systems and make them active through the nose as well,” Saltzman said. “One of the advantages we have is that the PACE polymer that we're using seems to be much milder, and much better tolerated in the lung than lipid nanoparticles are.”
A previous study from the Saltzman lab characterized PACE polymers with various chemical end-groups.
“There are a lot of chemistries that govern the transfection efficiency of mRNA, and we chose the top candidates and then tested them for our work,” said Hee Won Suh, an associate research scientist in the Saltzman lab, and co-corresponding author of the paper.
It’s a process that took a fair amount of trial and error.
“We have guiding design principles, but it was not perfectly understood which formulation would be best,” said Alexandra Suberi, a PhD student in the Saltzman lab and lead author of the paper. “We screened formulations with varying amine structures and polyethylene glycol content to find a formulation that would work well in lung cells. The end-group and PEG content both had a large effect on protein expression.”
The next step, the researchers say, is to test the delivery system for other therapeutic applications.

