For more efficient fuels, researchers take a much closer look at molecules
Producing carbon-based fuels from renewable sources such as wind or solar power is an important step towards a carbon-neutral economy. To make this kind of fuel production economically viable, though, researchers need to develop and optimize new catalysts that can yield renewable liquid fuels more efficiently. Doing so requires knowledge of unprecedented detail of the catalyst’s properties.
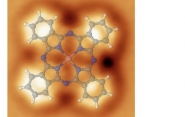
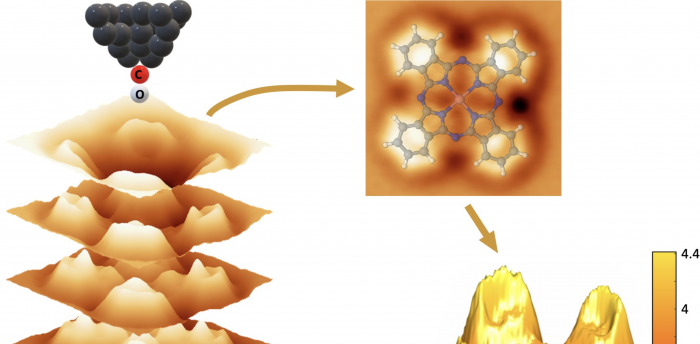 To that end, Yale researchers are using a technology known as noncontact atomic force microscopy that can generate three-dimensional images of the surface of molecules with an accuracy down to a picometer (about a billionth of a millimeter). The results are published in ACS Nano.
To that end, Yale researchers are using a technology known as noncontact atomic force microscopy that can generate three-dimensional images of the surface of molecules with an accuracy down to a picometer (about a billionth of a millimeter). The results are published in ACS Nano.
Better performing catalysts for renewable fuel production are critical to transportation sectors where batteries provide insufficient energy density, such as air travel, marine shipping, or long-distance trucking. Nanoparticle and bulk catalysts containing metals such as copper, zinc, aluminum, or chromium, which accelerate chemical reactions, have shown promise in enabling this type of fuel production. The problem, though, is making them economically viable, since the energy needed to drive the reaction is much higher than the energy produced from burning the manufactured fuel after it has been synthesized. That’s in part because of the limitations of bulk and nanoparticle catalysts.
Getting beyond this obstacle requires new catalysts that can yield renewable liquid fuels in a more efficient way. And to do that, researchers need much more detail about the catalysts’ properties.
Interactions between the catalyst and the reactant need to be “just right,” says Udo Schwarz, professor of mechanical engineering & materials science, who led the study. For instance, if you want a reaction to generate liquid fuel from carbon dioxide and water, an interaction that’s too strong will produce only hydrogen gas. But a weak interaction produces only carbon monoxide (CO), which acts as a reaction intermediary. Tuning CO adsorption strength – that is, how strongly they adhere to the catalyst’s active site – is key to achieving this tricky balance. However, the tunability of bulk or nanoparticle catalysts to achieve this “just right” interaction is significantly limited.
Catalysts made from a single molecule, though, are another matter. With molecular catalysts, researchers can subtly modify the interaction strength in multiple ways, such as by very slightly changing their chemical structure or by attaching them to different substrates. The number of potential modifications, however, is so vast that using a trial-and-error approach to identifying the most efficient structure-substrate combination is futile. To achieve meaningful improvements, researchers need to develop an intuition of what might happen if a specific change is implemented. And that requires a method for quantifying the effect such changes have on a single-molecule basis.
To do so, Yale researchers are using cutting-edge microscopy that can generate three-dimensional images of the surface of molecules with extreme accuracy: These images capture details down to a picometer (less than one 1/100th of the distance between two atoms) while locally quantifying the interaction strengths on a piconewton scale (a force of a piconewton is of the order of one 1/100th of the total adhesive force between two atoms).
Schwarz and his research team used this technology, known as noncontact atomic force microscopy, to get a detailed look at the surface of a molecule of cobalt phthalocyanine (CoPc). Hailiang Wang, professor of chemistry and a co-author of the paper, recently identified CoPc as a promising catalyst for methanol production. The cobalt sits at the center of the cross-shaped CoPc molecule, acting as the catalytically active site.
“They discovered that CoPc is a prime candidate for the efficient production of renewable fuel,” Schwarz said. “It's one of the most efficient catalysts to make methanol out of CO₂ and water. And once you have methanol, you can make all sorts of other popular fuels.”
Wang’s group has already shown that modifying standard CoPc by attaching amino groups (a nitrogen atom and two hydrogen atoms) to each of their four ends can further improve efficiency. Exactly why this happens, though, is unclear. Figuring this out, Schwarz said, requires extreme accuracy.
“We have developed this experimental procedure where we can have spatial mapping of the molecule’s force field above its surface, which we call three-dimensional atomic force microscopy,” he said, adding that it provides enough information to completely reconstruct the molecule’s interaction potential as well.
The technology features an oscillating cantilever with a sharp tip featuring a single atom at its end attached to an individual carbon monoxide (CO) molecule. By tracking the oscillating cantilever’s movements while its CO-functionalized tip is near the cobalt phthalocyanine’s surface, researchers can measure the force that the CoPc exerts on the CO, allowing them to make conclusions about binding strength.
Schwarz said the goal of the research is to establish a method that provides unprecedented insight into how strongly molecules adhere to molecular catalysts. With such detailed measurements, it will be possible to fine-tune the molecular structure and the nature of the material it’s deposited on for optimum performance as a catalyst.
The researchers plan to further develop the methodology to visualize even subtle changes in related properties such as electron density, orbital structure, or charge transfer to or from the support. They can then use the results for comparison with simulations and averaging macroscale methods.

