How do wounds heal? A fruit fly's wing offers clues

How long it takes for cells to rush to close a fruit fly’s wound can tell us a lot about the healing process in the early developmental stages of humans, and potentially lead to treatments for congenital disorders and prevent long-term damage.
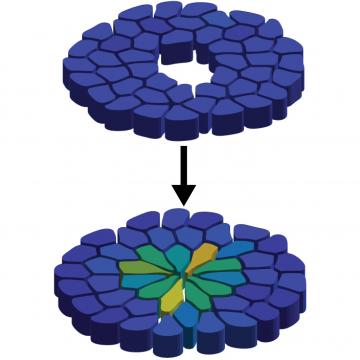
For a study published as a letter in Physical Review Research, a research team led by Prof. Corey O’Hern used previously published experimental data to develop computer simulations to describe how cells respond to wounds in fruit fly wings at the embryonic and larval stages. In both stages, extracellular matrix forms encircle the wound, and then shrink - pulling on the cells like a purse string to close the wound. It turns out that in the embryonic stage, though, wound healing happens a lot quicker than in later stages.
“What we noticed is that at the earlier developmental stage the wound closes much faster,” said O’Hern, professor of mechanical engineering & materials science, physics & applied physics.
That shorter response time, the researchers believe, is a life-preserving effect. That is, a wound that goes unhealed for a longer amount of time at an earlier stage might lead to big problems later on during development.
“A long time lag in wound healing could cause developmental delays and dysmorphic tissue changes,” he said. “Whereas in later stages of development, perhaps the organism can tolerate the wound over longer times and still develop normally.”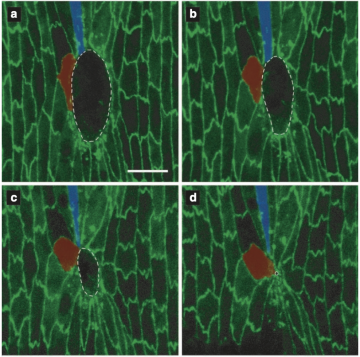
But the speedy healing process comes at a cost. The researchers compared the conditions of the healed tissues in the embryonic and larval stages, and found that the cells in the earlier, embryonic stage are more elongated, leading to flower-shaped healed wounds.
“When the purse string closes rapidly, the cells have to stretch,” O’Hern said. “The cells are all adhered to the edges of the purse string, so when the purse string shrinks and closes the wound, the cells must stretch. And this rapid closure causes the cells near the wound to become much thinner and elongated than they were originally.”
The cells also stretch during the healing process in the larval stages, but with more time to do so, they can relax back into their original shapes.
A key to this discovery was the computer model the researchers developed for the study. Most computer models of epithelial tissues - the outer regions of organs - consider the tissue as a single entity. The O’Hern team’s model treats the tissue as a collection of individual, deformable cells.
“So with our new model we can ask ‘How stiff is each cell? How mobile is each cell? And what's the shape of each cell?' - all independent of the other cells. Prior models have treated epithelial tissue as a single object with each cell strongly coupled to all other cells.”
This new research leads to new questions. For instance, the researchers don’t know the specific microscopic mechanism that causes the cells to return to their original pre-wounded shapes. Additional research can identify the chemical signaling process and associated biophysical mechanism that triggers the cell shape relaxation. More research in this area, O’Hern said, could lead to the development of a drug or other stimulus that helps human embryonic cells return to their original shapes in response to wounding as they do in later developmental stages, which would have important implications for possibly healing congenital disorders.

