Dr. Judy Cha Observes Crystallization in Real Time, Results In Nature Communications

The many uses for nanoscale metallic glasses include bioimplants and antibacterial applications. But questions about their properties have kept researchers from finding the best way to process these materials.
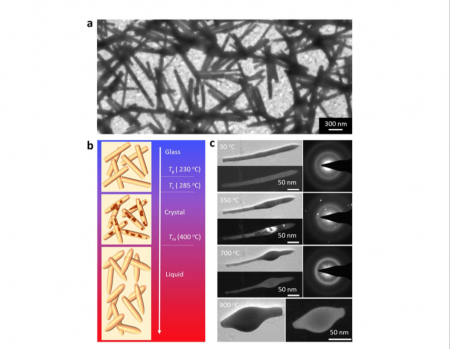 A team of researchers at Yale has now filled a critical gap in that understanding by observing in real time the nucleation of metallic glass nanorods. Their results are published today in Nature Communications.
A team of researchers at Yale has now filled a critical gap in that understanding by observing in real time the nucleation of metallic glass nanorods. Their results are published today in Nature Communications.
Using a transmission electron microscope (TEM) with in-situ heating of nanorods, the researchers can visualize the nucleation and crystallization of these materials at unprecedentedly small length scales.
“We needed to make the samples very small, so we could directly see with TEM and we’re one of the few groups in the world to do this,” said Dr. Judy J. Cha, Assistant Professor of Mechanical Engineering & Materials Science, lead author of the paper. “And we needed a very good in situ technique, to look at these materials while we heat them inside TEM.”
Previously, crystallization could only be inferred with the use of x-ray diffraction or differential scanning calorimetry. Neither allow for direct observation at the atomic scale, said Cha, who is also faculty at the Energy Sciences Institute at Yale's West Campus.
“You’re always looking at the aftermath of what happens,” Cha said of those techniques. “What’s exciting about our approach is that we now have a way to directly watch nucleation and crystallization of amorphous materials at a very high magnification in real time. There’s a lot we can study from this - this is just the start of this whole thing.”
Heated to a certain degree, atoms in these materials will begin to gather around a particle, a phenomenon known as nucleation. Once it starts, other atoms in the material also line up around the particle in an orderly fashion. That process is known as crystallization, and it’s what people working with metallic glass want to avoid. Once it’s crystalline, it loses the strong, pliable properties that make the material so useful.
Jan Schroers, a professor of Mechanical Engineering & Materials Science and one of the authors of the paper, said much is already known about crystallization, but nucleation remains very much a mystery.
“The early stages of this process are the most puzzling – yet, they determine the path,” said Schroers, a faculty members with Yale’s Center for Research on Interface Structures and Phenomena. “It’s a big open question in the solid state physics community, and now we have a tool to look at the very, very early stages of nucleation.”
That’s important, because it’s crucial when working with metallic glass to heat the material enough that it can be processed, but not so much that it crystallizes. When working with nanoscale materials, it’s particularly tricky because researchers didn’t know when nucleation begins at that size. Because of that, researchers have had to rely on parameters set for bulk materials. These parameters provide approximate - but not exact - guidelines for how hot nanomaterials can get before the onset of nucleation.
“If you’re making millimeter-scale samples, the bulk parameters that have been reported are good values to use, but if you’re doing nanomanufacturing, then our study tells us that they aren’t reliable,” said Cha. “We’re basically providing a guidebook that people can look at and say, ‘Now I have to change my parameters this way to ensure that these materials remain glass.’”
One thing they found was that the temperature at the onset of nucleation varied according to the size of the nanorod. Shape also affected the process; the crystallization process briefly stopped in the narrow middle section of one nanorod. Cha compared it to traffic congestion, with the atoms slowing down when space becomes more confined.
“This is the first time we are seeing this,” Cha said. “With TEM, we can look at just one metallic glass nanorod, and I can directly see things crystallize and the morphology changes of the sample at the same time, which is very powerful.”
The study was funded by the National Science Foundation’s Division of Materials Research.

