In A Different Vein

This story originally appeared in Yale Engineering Magazine.
It was her experience as a physician in the intensive care unit that pointed Laura Niklason in the direction of making engineered blood vessels for kidney dialysis patients. She worked with countless patients requiring needle injections multiple times per week, whose veins weren’t up for the job.
“Some patients had failures over and over and over,” said Niklason, the Nicholas M. Greene Professor in Anesthesia and Biomedical Engineering. “They’re in the operating room all the time and they get infections and they get hospitalized for those infections. And it’s just miserable.”
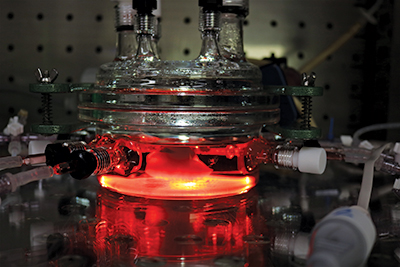 Off-the-shelf engineered blood vessels, on the other hand, could operate the same way that natural ones do but with the durability to withstand the abuse that dialysis requires.
Off-the-shelf engineered blood vessels, on the other hand, could operate the same way that natural ones do but with the durability to withstand the abuse that dialysis requires.
Niklason has been working for more than 20 years on what’s known as bioengineered human acellular vessels (HAVs). They’re the product that Humacyte — the North Carolina-based company that Niklason founded in in 2004 — specializes in. In addition to dialysis patients, they could also prove to be life-savers for patients with cardiovascular disease. This year, the U.S. military began exploring the use of Niklason’s HAVs to treat soldiers who have been injured on the battlefield and need a blood vessel repaired.
Also this year, Niklason and Humacyte realized a major breakthrough: In one of the longest follow-up studies of its kind, researchers found that their specially bioengineered blood vessels evolved into living tissue after human implantation. Beyond the immediate benefits, this transformation from non-living to living is a great sign for the future of regenerative medicine in general.
The results, which were published in Science Translational Medicine, showed that the vessels created in her lab — devoid of any cells when they were implanted in patients — had taken on cells, transforming the structures into living tissues that could transport blood and self-heal after injury. The implants stimulated the recipients’ cells to repopulate the vessels and take on all the characteristics expected of cells. They had, essentially, become the patients’ own blood vessels.
“For me that’s very exciting — we’re starting off with a nonliving implant and turning it into living tissue,” she said. “We think it’s a reason why these vessels work better than synthetic ones, which never become the patient’s own tissue, but remain a piece of plastic with a few scar cells on top.”
The HAVs for dialysis patients are 42 centimeters long, 6 millimeters in diameter and strong enough to endure a strong tug. Once implanted, they function like arteries from the get-go, and can be repeatedly punctured with needles.
Starting more than six years ago, the researchers began implanting the HAVs in patients with end-stage kidney disease. These patients typically receive hemodialysis three times a week. The procedure, in which a machine cleanses the blood of waste, salts and other materials, is often administered through a graft made from a synthetic material such as Teflon. These grafts, implanted in the arm, carry a high risk of infection, clotting, and scarring so much that the vessel closes up. Up to 40 percent of these grafts fail within one year.
An Astounding Idea Becomes Reality
It was in the mid-1990s when Niklason decided that this was the field she would focus on.
“I wanted to do something interdisciplinary — medicine, engineering, and even some math, where I could combine what I knew about biology and the physical sciences,” she said. “I also wanted to work in a field that was new, that wasn’t densely populated by many investigators. And tissue engineering at the time certainly fit that bill!”
In fact, she got a lot of confused looks when she told people her plans.
“People laughed at me! They thought it was so funny that I was trying to grow arteries in jars.”
 More than 20 years later, the idea of engineering organs still astounds. But with a remarkable amount of progress, Niklason has brought this once far-fetched notion far along enough to give hope for many patients, including those with kidney failure, heart disease, or in need of certain organ transplants.
More than 20 years later, the idea of engineering organs still astounds. But with a remarkable amount of progress, Niklason has brought this once far-fetched notion far along enough to give hope for many patients, including those with kidney failure, heart disease, or in need of certain organ transplants.
She came to Yale in 2006 and quickly gained a reputation as a valuable collaborator. Anjelica Gonzalez, an associate professor of biomedical engineering who also focuses on engineered tissue, said she was “awed and inspired” before meeting Niklason by her ability to apply engineering to advance medicine. Once they began working on various projects, Gonzalez said, she was even more impressed.
“As a mentor and collaborator, she is generous with her time, knowledge and resources,” Gonzalez said. “This underscores the idea that she is intensely dedicated to the advancement of science to improve human health.”
Niklason’s work with engineered tissue began with blood vessels, and about 12 years ago she added engineered lungs to her research. Walking through her bustling laboratory in the Amistad Building on a Monday morning, she stops to point inside a bioreactor to show off an engineered vessel. It sits on a rotating platform that allows it to achieve a gas exchange — essentially, to breathe.
A few aisles over, she points to another bioreactor, this one housing a set of rat lungs. Because a lung is a much more complicated tissue, it requires a much more complicated set-up. Numerous tubes run from the lungs to the machine, each facilitating a different function that a lung would naturally do on its own. There are pumps to control arterial perfusion and oxygenation, and one to essentially feed it and remove waste at the same time.
“It’s our attempt at artificial homeostasis,” said Micha Sam Raredon, a graduate student in Niklason’s lab. “We’re trying to control the whole milieu.”
Both the lungs and the vessels rely on the use of donor cells to repopulate the tissue, but the key is getting the cells to act accordingly once they’re there. In the case of lungs, that’s particularly tricky. “It’s not mature or functional now — that’s what we’re trying to get to,” she said of the engineered lung. “It’s living, but it’s disorganized.”
In 2010, Niklason’s lab successfully transplanted into a rat an engineered lung that was able to take in oxygen and breathe out carbon dioxide. It was a major milestone in the field. Getting a functioning engineered lung in a human, though, is complicated and comes with much higher stakes. Reaching that goal, she figures, is about 15 to 20 years away.
Off-the-shelf blood vessels, on the other hand, could be available in just a few years. “There’s a lot of caveats and stuff that we can’t control — how the trials turn out and what the FDA thinks, for instance — but we would hope to be on the market by 2021.”
How They Work
Years ago, Niklason had originally conceived the HAVs for use in heart bypass procedures. But she also considered the realities of the FDA approval process, and the clinical trials required for such a high-risk procedure as a heart bypass — as if growing brand new tissues in the lab wasn’t already fraught with its own complications. Instead, they decided to concentrate on using them for grafts for dialysis patients — a direction with lower patient risk, and one that would allow them to move forward quicker. “It was a way that we could study these things safely but also in a way that had a big potential for benefit.”
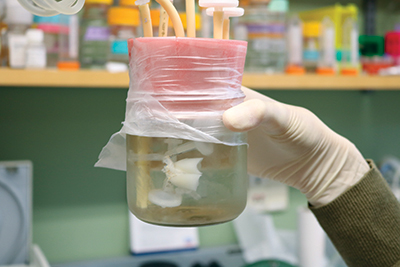 The HAVs are formed by placing human donor cells from smooth muscle tissue onto a tubular scaffold. They remain in a bioreactor for eight weeks, growing in vitro into an extracellular matrix, which is the structural support for the cells. All cells are then removed and the structures are cleansed of anything that could trigger an immune response. What’s left are mechanically robust, rope-like structures made from collagen and 40 to 50 other proteins in the matrix, a makeup that’s pretty consistent among humans — that’s key to why the patients’ bodies accept the vessels.
The HAVs are formed by placing human donor cells from smooth muscle tissue onto a tubular scaffold. They remain in a bioreactor for eight weeks, growing in vitro into an extracellular matrix, which is the structural support for the cells. All cells are then removed and the structures are cleansed of anything that could trigger an immune response. What’s left are mechanically robust, rope-like structures made from collagen and 40 to 50 other proteins in the matrix, a makeup that’s pretty consistent among humans — that’s key to why the patients’ bodies accept the vessels.
“We use someone else’s cells to make this great matrix and then take the cells away and implant that into the patient,” she said. “The patient doesn’t know it’s not him, so his cells repopulate that tissue.
In some ways, it’s like tricking the new cells into believing that this has always been their home.
“I think of the extracellular matrix being the apartment building, and this apartment building has the right ceilings and wall color, so that when cells crawl in, they say ‘OK, this is a blood vessel — I’m supposed to behave like a blood vessel cell.’”
Niklason’s company Humacyte is currently finishing up a Phase 3 trial, which compares the HAVs to synthetic grafts. The researchers are also enrolling for a second Phase 3 trial in which the HAVs will be compared to arteriovenous fistulas, another procedure used for dialysis in which an artery is connected to a vein.

