A Close Look Gets Answers About Water Filters

At the nanoscale, many things don't act as they do at larger sizes. That’s the case with certain membranes used for water desalination. A team of researchers set out to find out why.
In recent years, membranes have emerged as a promising way to provide crucial supplies of clean drinking water. Further improving the technology, though, requires advancements in the materials and processes. But those efforts are hindered by a lack of understanding about the relationship between membrane materials and the water and salt they're filtering.
 Researchers in the lab of Menachem Elimelech, the Roberto C. Goizueta Professor of Chemical & Environmental Engineering and the Environment, and at MIT addressed this knowledge gap by shedding light on the mechanisms that guide the behaviors of the films used in these membranes. The results were published Thursday in the Proceedings of the National Academy of Sciences (PNAS). The work came out of the Center for Enhanced Nanofluidic Transport (CENT), an Energy Frontier Research Center established by the U.S. Department of Energy that’s based at MIT. Elimelech is one of the principal investigators at CENT.
Researchers in the lab of Menachem Elimelech, the Roberto C. Goizueta Professor of Chemical & Environmental Engineering and the Environment, and at MIT addressed this knowledge gap by shedding light on the mechanisms that guide the behaviors of the films used in these membranes. The results were published Thursday in the Proceedings of the National Academy of Sciences (PNAS). The work came out of the Center for Enhanced Nanofluidic Transport (CENT), an Energy Frontier Research Center established by the U.S. Department of Energy that’s based at MIT. Elimelech is one of the principal investigators at CENT.
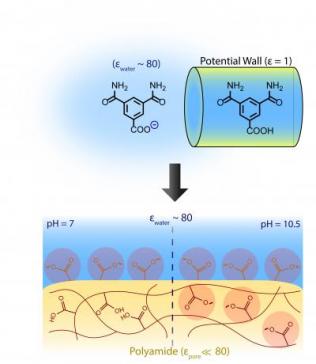
State-of-the-art membranes used for water purification processes such as desalination leverage have nanoporous polyamide thin-films to filter out impurities. Polyamide ionization, in which electrically neutral molecules in the polymer gain or lose electrons to become electrically charged, is key to the separation of charged solutes. But because of confinement effects - that is, the unexpected phenomena that happen at scales of less than 2 nanometers - not all ionizable molecules get charged.
“Right now, what that means is that a lot of the membranes we’re using aren't operating at their full potential,” said Cody Ritt, lead author of the paper and a Ph.D. candidate in Elimelech’s lab.
Finding out exactly what is happening at this scale has been an elusive goal within the field because the membranes are so thin - 10 to 200 nanometers - that classic measurement techniques typically are not applicable. “So it took a combination of several approaches for us to extract that information,” Ritt said.
One approach the research team took was to create computer models. “These are very difficult-to-understand materials because everything that's going on is happening at the nanoscale,” said co-author Heather Kulik, associate professor of chemical engineering at MIT and a principal investigator at CENT. “Something unique is happening to the water under confinement, but how do you understand that? So what my lab did was build models of that confinement.”
As a result of their analysis, the researchers found that completely ionizing polyamide membranes would increase their selectivity and significantly improve the efficiency of the desalination process.
Co-author Jay Werber, a postdoctoral associate at the University of Minnesota’s chemistry department, said the research could have long-term benefits by adding to the basic understanding about these materials.
“Any fundamental knowledge that we could gain on how these things work and why they work so well could enable the design of better membranes in the future,” said Werber, a former Ph.D. student in Elimelech’s lab.
Elimelech said the work could go a long way toward significantly improving membranes and getting closer to the kind of technology needed to combat global water scarcity.
“This is the kind of research that requires a collaboration of different expertise, which was made possible through the establishment of CENT,” he said.
Also contributing to the study are Mengyi Wang and Zhongyue Yang at MIT, and Yumeng Zhao at Yale.
Water image by Davide Restivo.

