Amir Haji-Akbari Wins Career Award For Work With Crystalline Materials

Amir Haji-Akbari, assistant professor of chemical & environmental engineering, has received a Faculty Early Career Development Program (CAREER) Award from the National Science Foundation (NSF).

The $500,000 award is for Haji-Akbari’s proposal, aimed at using advanced computational techniques to develop a better process for making single crystalline materials, which are in high demand in a number of industries. The prestigious NSF CAREER award is designed to support junior faculty who exemplify the role of teacher-scholars.
Because of their unique properties, single crystalline materials are very useful for a variety of applications in electronics, solar energy conversion and aerospace industries. Making single crystalline materials, however, is very difficult. Most solids are crystalline - that is, their constituent atoms and/or molecules arrange themselves into a periodic structre. But usually, these materials are polycrystalline - they have periodic regions with different orientations. Single crystalline materials are in such demand because they usually have superior transport properties. One advantage of these properties is that they make these materials excellent conductors.
“Single crystalline cells have a considerably higher efficiency than the polycrystalline ones,” Haji-Akbari said, adding that one reason for the difficulty of making single crystalline cells is due to the mechanism of crystallization – that is, how a material freezes into its solid phase. The outcome of nucleation - the first and the most critical step in crystallization - is very susceptible to exterior conditions, such as temperature, pressure and concentration.
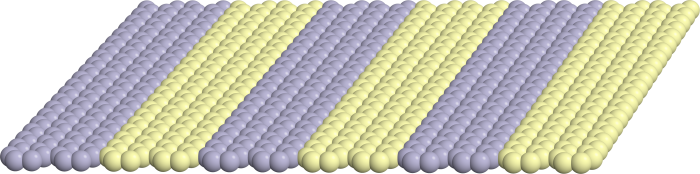
“These variables are very difficult to control, even in the lab, let alone in a large-scale industrial operation process,” he said. “That’s why you get polycrystalline materials most of the time - because you can’t control when nucleation occurs, or where it occurs.”
Because of this difficulty, producing single crystals is usually conducted in two steps in industry settings, with the mass scale production of the single crystal occurring in the second stage, via crystal growth from an existing single-crystalline seed.
Haji-Akbari is working to modulate the nucleation and growth of single crystals by studying the effects of engineered surfaces, called crystal nucleating agents (CNAs), used to accelerate the nucleation and growth of single crystals. He will look at how nanoscale patterns of a CNA surface affects crystal nucleation and identify the operating conditions that lead to the reduced sensitivity to changes in operating variables.
“Most materials are structurally uniform, at least as far as crystallization, which happens at the nanoscale, so I’m trying to use nanopatterning of the surface,” he said. “The goal is to have materials that don’t have that sensitivity to, say, temperature or concentration.”

