Stickiness: More Complicated Than We Thought
The Post-it notes that line your computer monitor may seem pretty commonplace, but it turns out that there's a lot more going on than we realized when it comes to making them stick.
For decades, researchers in the field of contact mechanics thought they had a good handle on how certain types of materials make adhesive contact. But a new study from the lab of Prof. Eric Dufresne, published in the Proceedings of the National Academy of Sciences, finds that the classic model doesn't always apply. The results have potential for more than just a better sticky note; it could be used to advance the field of medical implants and other applications.
The classic theory of contact mechanics, first posited in the 1970s, describe adhesion as a competition between adhesion energy and elasticity. Adhesion energy drives materials to try to conform to each other to make as much contact as possible, and elasticity resists the deformation required to make that happen.
However, these theories don't account for surface tension, which is a factor when it comes to the very soft materials observed in the PNAS study. Surface tension adds an additional resistance to deformation on top of the resistance provided by elasticity, by causing the material to resist any stretching that creates extra surface area. In a similar phenomenon, surface tension causes liquid droplets to stay in spherical shapes that minimize surface area.
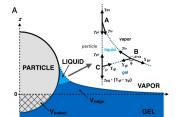
The researchers looked at what happens when rigid glass spheres, seven to 32 micrometers in radius, come into contact with a silicone gel. For the most part, a gel can be considered a single, homogenous material. But the researchers found that, at the area of contact between the gel and the spheres, the silicone separates into two phases. Even though fluids don't separate from silicone easily, some of the liquid comes out from the interior of the gel. This means that what was always thought of as a three-phase contact line — between air, the rigid solid [sphere], and the soft solid [gel] — is actually a four-phase contact zone in which air, the rigid solid, the gel and the liquid from inside the gel all meet.
"So you get an example of every type of contact line that we know about, or study," said Dr. Katharine E. Jensen, lead author of the paper, and a postdoctoral associate in the department of Mechanical Engineering & Materials Science. "Instead of having this single contact, as we've always been drawing this situation for 40 years, what we're seeing now with the gels is you actually get a four-phase contact zone with three contact lines."
Although the results go against conventional thinking, she said, they also make intuitive sense. "I've had a lot of people in the field say 'I never would have thought it would have done that, but now that you've shown it, of course it does that.'"
The next step, Jensen said, is to see what happens in similar experiments when a force is pulling the objects apart. "The engineering of adhesives is about how long do you want it to stick, how well do you want it to stick, and how heavy of an object can it hold? These are all thing you worry about in designing adhesives."

