Shedding Light On A Mutation In The Heart

Every February is American Heart Month, designed to raise awareness about the causes and treatment of heart disease. Before it winds down this year, let's highlight some SEAS research from the lab of Stuart Campbell, assistant professor of biomedical engineering & physiology, which sheds some light on genetic mutations and their impact on heart disease.
 Campbell's lab used computer simulations to look at the interaction of actin and myosin, two proteins that need to bind with each other for the heart to function properly.
Campbell's lab used computer simulations to look at the interaction of actin and myosin, two proteins that need to bind with each other for the heart to function properly.
Calcium is typically thought of as being the master regulator of actin-myosin binding in cardiac muscle – when it is present, the muscle contracts. Campbell and his co-authors wanted to understand what would happen if this switch mechanism was assumed to be less-than-perfect, allowing some actin-myosin interactions to occur even in the absence of calcium. They found that including some 'calcium-independent activation' (CIA) actually made the model behave more realistically. Campbell suspected that the source of this 'leaky' switch behavior was an interaction between actin and troponin I – a protein that helps block myosin binding.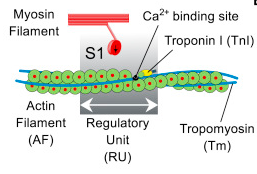
"To confirm that hypothesis, we needed to find a mutation to troponin I that was likely to weaken its binding to actin," Campbell said. "We found a clinical report of a mutation (R145G) that causes cardiac muscle disease, and since it occurs precisely in the region of the protein known to bind actin, it seemed like an excellent candidate." The team also found published data showing a defect in heart relaxation when R145G was engineered into mice. Using the new model, they demonstrated that this slowed relaxation could be explained just by increasing the amount of CIA. In other words, the computational model explained in quantitative and mechanistic terms the effects of the troponin I R145G mutation.
"We spent a lot of time updating this model, so it was exciting to see it succeed in this way," he said. "This is the first time that I'm aware that a simulation has been used to capture the consequences of a disease-causing mutation on muscle twitch."
Ideally, he said, this computational model will be used to analyze other mutations that cause cardiac muscle disorders.
For more about the results of Campbell's study, go to Biophysical Journal.

