The Science of Membranes' Gatekeeping Abilities
Controlling what passes through membranes - whether it’s ions or the microscopic components of a solution known as solutes - is crucial to numerous applications, from purifying salty water with filters to developing new medicines. It relies on their semipermeability - that is, their ability to let some things pass while blocking others.
“One of the questions we have for designing membranes for separation processes is increasing the ability to predict the extent to which they’re selective to different types of solutes, molecules or ions in the mixture,” said Amir Haji-Akbari, assistant professor of chemical and environmental engineering.
But designing membranes to do exactly what you want is tricky. That’s because the ways that the different components of a solution pass through ultra-selective membranes are complicated by the variations in how long it takes certain entities to pass through them. For instance, the time for a sodium ion to pass through a membrane with positively-charged pores will be much longer than that of a chloride ion. As a result, conventional molecular simulations generally aren’t up to the task of capturing the full picture, particularly when the passage times are longer than a microsecond.
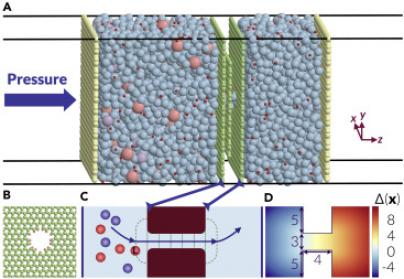 Using a recently-developed technique, known as “jumpy forward flux sampling,” Haji-Akbari has developed a way to accurately and efficiently compute long passage times. The technique allows researchers to accurately account for the progress of an ion or solute, even though the patterns of the movements can change a lot over a short period of time. The results are published today online by Matter.
Using a recently-developed technique, known as “jumpy forward flux sampling,” Haji-Akbari has developed a way to accurately and efficiently compute long passage times. The technique allows researchers to accurately account for the progress of an ion or solute, even though the patterns of the movements can change a lot over a short period of time. The results are published today online by Matter.
“It will allow us to probe much wider parameters for designing good membranes,” he said. “For example, you can change many different things - pore sizes, the shape of those pores, chemical functionalization, mechanical properties, conductivity - and these changes can result in large variations in mean passage times for the solutes you want to reject.”
That’s because the method they’re using allows them to efficiently calculate passage times for a particular membrane under particular conditions.
“If you want to design a membrane that allows passage of chloride ions more than nitrate ions, for example, then the ability to calculate these passage times efficiently is very valuable.”
By applying this method to a system in which sodium and chloride ions traveled through a nanoporous membrane made of graphite, the researchers also identified the two factors that impede the leading ion’s passage through the membrane. In one of these factors, first discovered in this work, the remaining ions are pulling the leading ion back with static electricity. It’s the result of the leading ion’s tendency to cause the oppositely charged ions to build up at the pore entrance. The other factor is the widely known phenomenon of partial dehydration, in which the leading ion loses some of the water molecules that have strongly bound to it, allowing the ion to fit inside the pore. This inhibits solutes and other ions from passing through nanopores.

