Breathing Easier with Cell Differentiation
By 2020, lung disease is expected to become the third leading cause of death worldwide, in part because damaged lung cells do not regenerate. But new research from a team of Yale scientists led by professor of anesthesiology & biomedical engineering Laura Niklason confirms that some human stem cells can trans-differentiate into lung epithelial cells, an ability that could be used for lung cell therapies that counteract lung damage or for bioengineering lungs for transplant. However, the source of the stem cells, as well as the medium used for culturing the cells, significantly affects the differentiation results.
The stem cells being researched, known as mesenchymal stromal cells (MSC), were transplanted into rat lungs that had undergone decellularization, a process that removes all the cells from an organ while retaining the proteins and fibers that give the organ structure. This retained “extracellular matrix” can be used by bioengineers and medical researchers as a scaffold that promotes cell growth into a desired architecture while also directing the differentiation of the cells into the desired cell types.
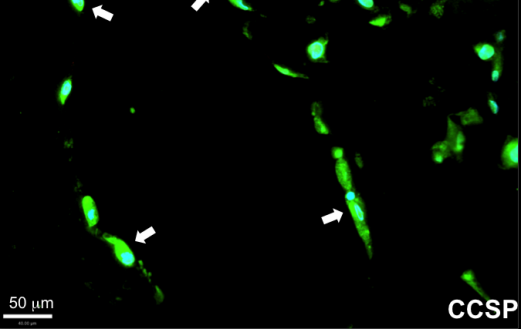
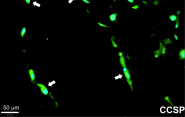
Niklason’s team showed that MSCs taken from human adipose tissue (commonly known as body fat tissue) and from human bone marrow can both differentiate into lung epithelial cells. However, MSCs from adipose tissue were capable of a broader range of differentiation and could attach to more locations in the decellularized lung; adipose cells were also capable of forming Club cells, a cell type that plays an important defensive role in breaking down airborne toxins. On the other hand, bone marrow MSCs were more limited in their application and appeared to repopulate only the distal lung, alveolar regions.
Notably, Niklason’s team—including postdoc Julio Mendez, associate research scientist Mahboobe Ghaedi, and assistant professor of surgery Derek Steinbacher—is the first to use human MSCs from adipose in repopulating decellularized lung tissue.
In addition to the effect of the origin of the transplanted tissue, a key finding of the research was in just how much the culture substrate influences transplanted cell differentiation. For example, culturing cells in fibronectin instead of collagen resulted in nearly twice as many cells that expressed CCSP, a protein associated with Club cells. Additionally, adipose MSCs cultured on human extracellular matrix formed a lattice-like network throughout the culture dish.
These results, published in Tissue Engineering Part A, lay a groundwork for better understanding the cell combination that produces the best results in lung tissue engineering.

