Optimizing Hydrogen Production from Biomass Gasification
For decades, hydrogen has been touted as a fuel of the future, providing a cleaner, more sustainable energy source that could one day replace fossil fuels. One promising and emergent method for producing hydrogen is direct gasification of biomass – producing gaseous hydrogen from renewable organic resources, ranging from crop and forest residues to animal or municipal waste.
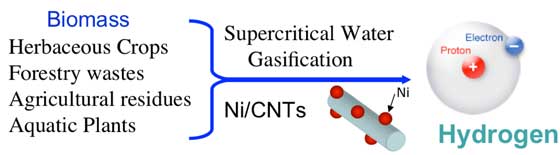 Not all biomass gasification methods are the same, however. One, which has been identified as particularly suited for biomass feed stocks with high moisture content, is supercritical water gasification (SCWG). "A primary advantage of supercritical water gasification," says André Taylor, assistant professor of chemical engineering, "is that it uses water as the reaction solvent, eliminating the need for drying the biomass and, thereby, increasing the overall thermal efficiency."
Not all biomass gasification methods are the same, however. One, which has been identified as particularly suited for biomass feed stocks with high moisture content, is supercritical water gasification (SCWG). "A primary advantage of supercritical water gasification," says André Taylor, assistant professor of chemical engineering, "is that it uses water as the reaction solvent, eliminating the need for drying the biomass and, thereby, increasing the overall thermal efficiency."
Researchers have recently begun using catalysts to further improve the technology. In the November publication of Applied Catalyst B: Environmental, Taylor, along with colleagues at the University of Michigan – Ann Arbor, propose a new method for synthesizing nickel-based catalysts that offers many advantages over traditional metal loading methods. These new type of catalysts may also offer improved performance over commercially available catalyst materials.
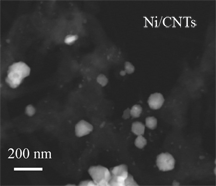 The "one-pot" synthesis method using supercritical methanol was first introduced by Taylor as a means for improving platinum loading on 1-D carbon supports for fuel cell development (see Another Step Forward for Fuel Cell Technology). In this latest work, Taylor demonstrates that supercritical methanol can be used to efficiently and effectively load nickel catalysts onto inert carbon nanotubes without the adverse effects common in alternative metal loading methods – damage to the nanotube structure and production of hazardous waste.
The "one-pot" synthesis method using supercritical methanol was first introduced by Taylor as a means for improving platinum loading on 1-D carbon supports for fuel cell development (see Another Step Forward for Fuel Cell Technology). In this latest work, Taylor demonstrates that supercritical methanol can be used to efficiently and effectively load nickel catalysts onto inert carbon nanotubes without the adverse effects common in alternative metal loading methods – damage to the nanotube structure and production of hazardous waste.
Upon testing their synthesized catalysts in the supercritical water gasification of cellulose (component of woody biomass), researchers were able to demonstrate both similar hydrogen yields and a higher hydrogen to methane gas product ratio compared to that of commercially available catalysts.
"If the goal of gasification is hydrogen production," says Taylor, "optimization of a catalyst with a preferred product ratio could be useful in understanding which mechanisms prevent hydrogen atoms from getting tied up in the formation of methane."
Read the advanced on-line journal publication at Science Direct.

