New model of connective tissue opens door to inflammation research
Biomedical engineers at Yale University have developed a new model for studying how an organ’s inflammation is affected by nearby nonliving connective tissue, such as the collagen in the amniotic membrane, tendons, and cartilage. The group’s polymer-based model replicates nonliving human tissue while allowing researchers to control the mechanical and biochemical influences on the tissue.
“Our model provides a useful tool to investigate how inflammation is influenced by human proteins, integrin binding sites, mechanical cues, and other regulatory aspects of the body, but in a controlled lab setting,” says assistant professor Anjelica Gonzalez, principal investigator of the research, published May 7 in the journal TECHNOLOGY. “The tools previously available for investigating leukocyte migration were limited in their relevance to human tissue and weren’t easily manipulated for laboratory studies.”
Inflammation, the body’s response to harmful stimuli, occurs due to the movement of leukocytes (white blood cells) from the blood into the injured tissue through the vessel wall. The migrating leukocytes can sense and can be regulated by the nearby proteins and carbohydrates that comprise the body’s nonliving connective tissue, known as the extracellular matrix, which provides structure in the spaces between blood vessels and cells.
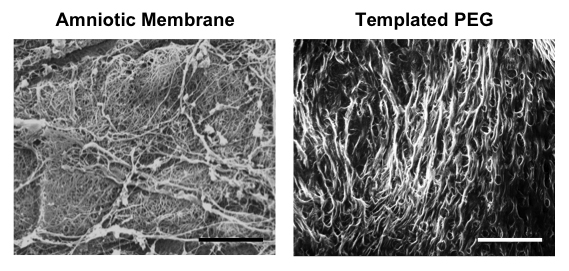
The Yale team developed a model of the extracellular matrix using polyethylene glycol (PEG), a polymer commonly used in medicine because of its compatibility with the body.
Although PEG is easily modified biochemically and mechanically, and is therefore commonly used in two-dimensional investigations, its small pores prevented it from being used for three-dimensional investigations. But using a technique called urea crystal templating, Gonzalez’s team can now reliably control the creation of complex pore networks throughout the gels.
“With PEG, not only can we replicate healthy tissue, but our ability to control the structural, mechanical, and biochemical signals sent to migrating leukocytes allows us to also replicate diseased tissue. So we can now investigate how inflammation is affected by disease,” says engineering doctoral student Holly Lauridsen, the paper’s co-first author along with research assistant Bryan Walker.
Using their model, Gonzalez’s team determined that higher local elasticity combined with small pores, high pore densities, and whole proteins promotes the likelihood of a certain kind of leukocyte migration, known as amoeboid migration, in response to chemical stimuli. Unlike other models, the Yale team’s model can control each of these four parameters independently to isolate which features best facilitate this type of leukocyte migration.
Moving forward, the team plans to augment their model by incorporating more complex proteins and biochemical signals.
“Because PEG is already commonly used in the medical field, countless methods exist for incorporating peptides and proteins into the gels,” says Walker. “Our model opens the door for these methods to be used in future inflammation experiments.

