New Model of Inflammation Helps Explain It, Suggests How to Ease It
Biomedical engineers at Yale University have developed a new way of studying inflammation, revealing the biological process as more complex than previously understood and offering new hints about how it might be treated.
Inflammation is the body’s response to harmful stimuli achieved by the movement of leukocytes (white blood cells) from the blood into the injured tissue through the vessel wall.
The Yale team, led by Anjelica Gonzalez, developed a model of inflammation that better represents how it works in complex, multi-cell human blood vessels. The model suggests that blocking the action of certain cells might be a way to minimize or prevent it.
To date, most inflammation research has relied on mouse models or human models with only a single cell type, the endothelial cells that line the interior surface of blood vessels.
“Inflammation research has basically been at a standstill for a few decades,” says Gonzalez, an assistant professor of biomedical engineering. “We understand really well all the molecular interactions in humans that lead to leukocytes getting stuck on the vessel lining, then crawling through the endothelial cells of the vessel wall and into the tissue. But the vessel is much more complex than just the endothelial cells.”
For example, if only the type of cell mattered, then the endothelial cells found in mice vessels would be ideal. But mice vessels rely on subtly different molecules than their human counterparts. This difference, according to Gonzalez, limits the usefulness of mouse models for significant advances in human research.
“So specific are the steps of what we call the leukocyte adhesion cascade that one molecule difference can really change the way we evaluate the whole process,” she says.
The narrow focus on a single cell type has similarly limited research with human models. When focusing only on the effects of endothelial cells, researchers miss how the one cell type’s leukocyte recruitment may be influenced by other cell types.
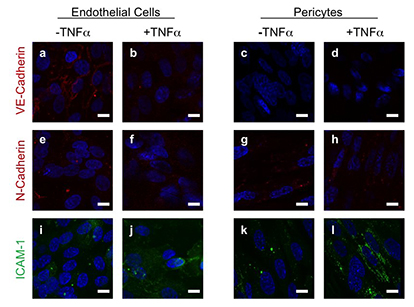
“Our model is composed completely of human cells, and we tested on only human cells,” says Gonzalez, whose team consists of Jordan Pober, Bayer professor of translational medicine at Yale, and engineering doctoral student Holly Lauridsen. “And it’s been engineered so we can evaluate how the cells signal each other through cell-cell contact, through release of soluble signals, or through deposition of matrix proteins, which are the connective proteins they use to talk to each other.”
Using their model, the team found that cells on the outside lining of the vessel signal the inside endothelial cells in a way that can inhibit inflammation. Chronic inflammation might therefore be caused when these cells are somehow modified during disease states, Gonzalez said. Also, when the two types of cells are together, one molecule in particular is much more effective than when the cells are apart.
“That was a really unique finding, one that means that molecule should be a target for inhibiting leukocyte migration into the tissue,” she said. The group published their research in The FASEB Journal in December.
Gonzalez is working to develop the complexity of the model further, which could pave the way for cell therapies that inhibit chronic inflammation by modifying the cells that make up the vessel itself.

